The Science Behind Why Everything Melts Eventually
The phrase "everything melts" might sound simple but it reveals an interesting scientific fact. Under the right conditions every solid can turn into a liquid. Melting isn’t just about ice cream on a sunny day or chocolate in your hand. It is a basic physical process that affects everything from the metals inside your gadgets to the plastics we use daily. Essentially melting happens when molecules gain enough energy to break free from their rigid arrangement and move more freely.
What Does Melting Mean? A Simple Explanation That Makes Sense
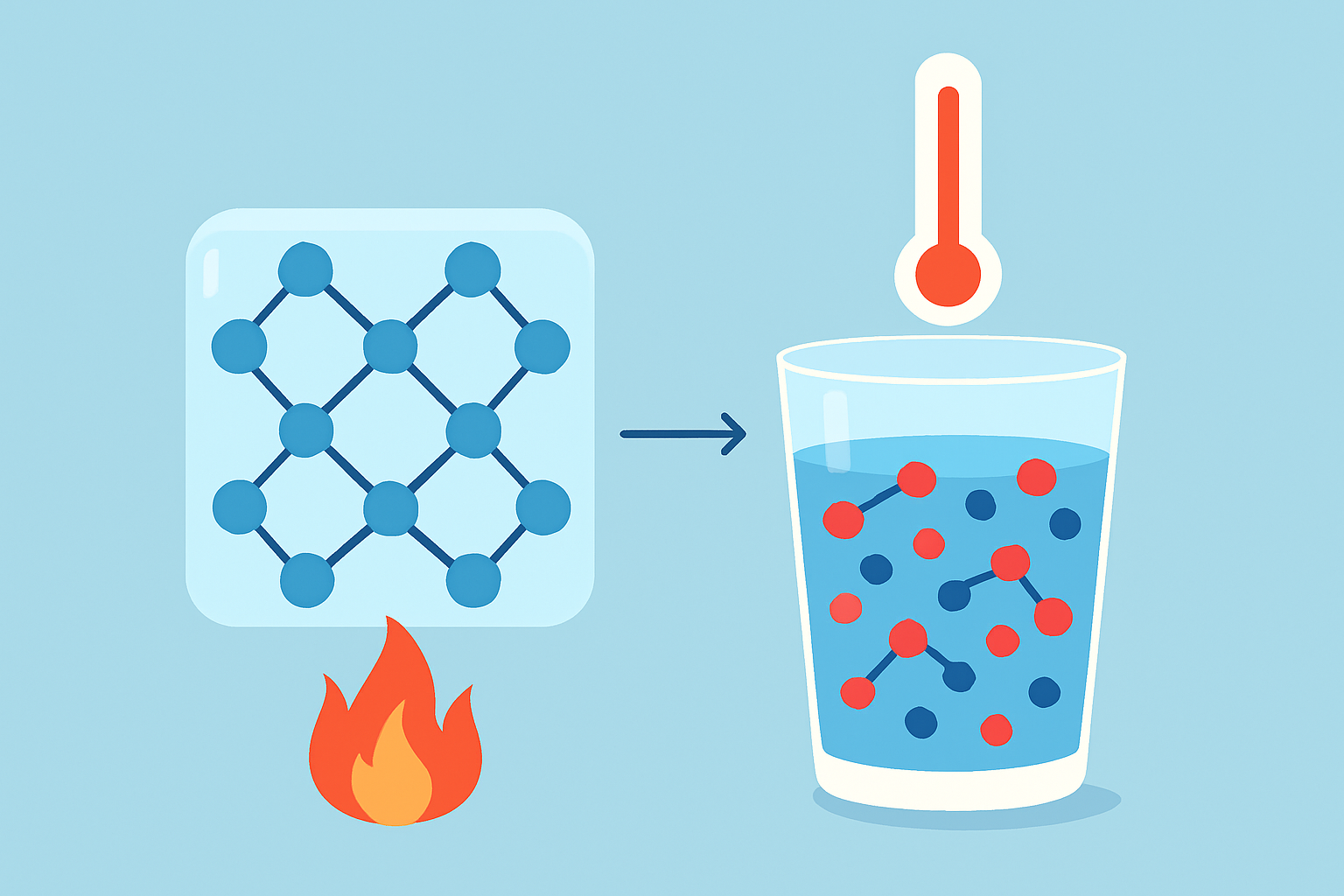
Melting is a phase transition where a solid gradually turns into a liquid as the temperature climbs. When solids get heated their molecules don’t just sit still—they start vibrating more vigorously until they break free from their fixed spots.
- Solids have their molecules snugly locked in fixed spots, but they’re never completely still. They keep vibrating away as if they’ve got a restless itch.
- When the temperature rises, these vibrations become so lively that they break free from the rigid bonds holding them tight.
- The melting point is basically the magic temperature when a solid decides to let loose and turn into a liquid, as long as the pressure allows it.
- During melting, the substance soaks up energy but the temperature remains steady. This sneaky bit of energy is called the latent heat of fusion.
- Melting is just one example of a phase change. It involves a change in how molecules are arranged and the energy they carry around.
Why Does Everything Melt? A Down-to-Earth Dive into the Science Behind It
When solids get a good dose of heat, they finally give in to the pressure and the forces holding their molecules together start to break apart. This turns the solid into a liquid.
Melting points mostly hinge on a material’s unique chemical makeup and the way its molecules are arranged. Some substances melt right on the nose at a precise temperature, while others tend to soften gradually over a range—usually because of impurities or wonky molecular structures throwing a wrench in the works.
| Material | Melting Point (°C) | Notes |
|---|---|---|
| Water / Ice | 0 | Pure ice doesn’t mess around — it melts exactly at 0°C |
| Iron | 1538 | A metal that really brings the heat with its high melting point |
| Gold | 1064 | That coveted precious metal that melts right on the dot |
| Polyethylene | 115-135 | Plastic that tends to soften over a range, thanks to its quirky structure |
| Sucrose (Sugar) | 186 | An organic compound that throws in the towel and breaks down when melted |
| Alumina (Ceramic) | ~2072 | Boasts an impressively high melting point due to a tough crystal setup |
The Role of Melting in Everyday Life and Our Lifestyle More Than Just a Science Thing
Melting sneaks into so many parts of our daily lives—from the simple joy of chocolate melting slowly on your tongue to the heavy-duty jobs like casting metal. It even shows up in the big picture, with things like polar ice melting that seriously shake up global climate patterns.
- Chocolate has this lovely habit of melting just right when it is warmed a little above room temperature, and that really brings out its creamy texture and rich flavor.
- When it comes to candle wax, how it melts is very important because it is the secret behind how candles burn smoothly and fill a room with their scent.
- Ice cubes do double duty in drinks. They chill your favorite beverage but also slowly water down the flavor, which some might say is part of the charm or the curse.
- Sunscreens aren’t just sitting pretty on your skin. Their ingredients soften or melt a bit in the heat, and that subtle change can affect how well they shield you from the sun’s rays.
- Metalwork is quite a dance of precision, with carefully controlled melting and solidification shaping and joining parts almost like a craftsman’s magic.
- Pollutants like asphalt or tar aren’t so straightforward either because they melt differently under environmental heat, and that quirky behavior influences just how much they impact the environment.
Understanding melting gives us a leg up when it comes to making smarter lifestyle choices. Properly storing food can be a real game-changer and helps it avoid the dreaded meltdown or spoiling too soon. Choosing the right materials for home repairs or gadgets means they’ll stand their ground better when the temperature takes a nosedive or soars. Wrapping your head around how ice melts isn’t just for science buffs—it helps protect the environment. For example, opting for plastics with higher melting points keeps them from warping in the heat. Having a grip on phase changes can make cooking smoother and often results in tastier dishes.
Common Misunderstandings and a Few Surprising Nuggets About Melting
Some people tend to believe that certain objects never melt, or that melting happens in the blink of an eye and always at a fixed temperature. Another common mix-up that I have seen time and again is confusing melting with dissolving. No solid is truly immune to melting, the melting point can actually shift depending on the circumstances.
- Metals do melt, but they usually require pretty high temperatures to get there which means they are not exactly out of reach.
- Melting is a physical change and different from burning or combustion so don’t confuse the two.
- Liquids don’t actually have a melting point, they have a freezing point where they turn solid instead.
- Substances don’t instantly change from solid to liquid right at the melting point. Instead, melting often takes its time and can occur over a range of temperatures.
"Melting is a bit like slowly unzipping an old zipper; the molecules that were once tightly clinging to each other begin to ease up, one by one, quietly allowing the solid to slip into a liquid state."
Key Factors Affecting Melting Pressure Purity and a Few Other Important Considerations
Melting points aren’t set in stone. They can shift around a bit depending on factors like pressure and the purity of the material. Sometimes, increasing the pressure can raise the melting point or lower it depending on the molecular structure.
Ice tends to melt at lower temperatures when pressure is applied—something you have probably seen on an ice rink where the skate blade’s pressure creates a thin sneaky layer of melt. Similarly, throwing salt on ice works like a trick to lower its melting point and makes road ice easier to tackle.
- Changes in pressure can nudge melting points up or down—metals tend to get a boost and ice often melts more easily because of how the molecules shuffle around.
- Impurities and alloys are like unwelcome party crashers in the crystal arrangement and usually cause the melting point to dip a little.
- When you zoom into the nano-scale, size really does matter. Smaller particles often melt at lower temperatures mostly because their surfaces have a bigger say in the game.
- Differences in crystal structures tweak the bond strength. This is the main reason why various polymorphs don’t all follow the same melting script.
How Scientists Dive Into Melting and Make Their Best Guesses
Researchers often turn to techniques like differential scanning calorimetry (DSC) to figure out exactly how much heat a material soaks up as it melts. This trick helps nail down melting points and latent heat values with surprising accuracy. Meanwhile, microscopy gives them a front-row seat to the melting show at micro and nanoscale levels and provides a fascinating glimpse into how the structure morphs during those phase transitions.
Advanced computational techniques like molecular dynamics simulations give researchers a clever way to peek into how new materials melt by modeling the dance of atomic interactions and the heat they absorb.
The Surprising Role of Melting in Technology and Climate Change
Understanding melting plays a important role in developing technologies like phase change materials (PCMs) that are becoming heroes in energy-efficient buildings and thermal storage systems. PCMs work by soaking up and releasing heat as they melt and solidify at specific temperatures, making temperature control less like guesswork. Melting metals precisely in 3D printing is transforming manufacturing by enabling the creation of intricate custom parts with far less waste. This opens exciting doors in medicine and aerospace.
Melting ice caps and glaciers have become a pressing concern in climate science, as everything melts at an alarming pace. Their retreat directly influences sea levels and ecosystems across the globe. Getting a solid handle on how these ice giants melt—and being able to predict their pace—gives policymakers and communities a fighting chance to brace for environmental challenges like flooding and habitat loss.





